What encourages us to eat? Factors that start and stop intake

- 1884
- 109
- Frederick Cormier
Several factors influence what time we eat, the amount we eat and when we stop doing it.
Content
Toggle- What begins the behavior of eating?
- What for the behavior of eating? (Satiety signals)
- Short -term satiety signals
- Long -term satiety signals
- Leptin function
- Where does the liberated leptin act?
- Insulin function
What begins the behavior of eating?
We have the tendency to consider that food is important only for physiological processes. That is, we eat when we need calories.
But we must keep in mind that Food is a relatively ineffective way to quickly get blood calories. To increase blood nutrient levels, food must be processed, digested in the stomach, have to pass the intestines and should be absorbed in blood.
Luckily, under normal conditions, it is not frequent that blood fuel levels are below those necessary to meet the needs of the different tissues. And therefore, We start eating when we still have important amounts of energy available.
Food behavior is very influenced by social and cultural factors. Many times we eat for habits or by the action of different stimuli of the environment such as the vision or the aroma of food, a clock that indicates that it is eating time, etc.
Many studies have shown that eating behavior can be conditioned classically and, therefore, any stimulus that has been associated with food intake, can cause eating behavior.
But intake also depends on metabolic factors.
If we skip meals, we will be more hungry, and surely this goes through the existence of physiological signals that indicate the decrease in nutrients from our long -term reserves. So, The use of long -term reserves could provide an indicator signal that is eating time.
It seems clear that the feeling of hunger is inversely related to the amount of nutrients excessively ingested in the previous food.
The brain responds to two types of appetite signals:
- Short -term signal: They are determined by the availability of blood nutrients and are detected by liver and brain receptors.
- Long -term signal: They originate in the adipose tissue that contains the long -term reserves.
When the reserves are full, a peptide hormone that has an inhibitory effect on the brain mechanisms that control the behavior of eating is segregated. When the level of this hormone is high, the brain becomes less sensitive to hunger signs in the short term that report the availability of nutrients, producing a decrease in intake.
Before a prolonged fasting period, long -term reserves decrease, and adipose cells decrease the release of this hormone, causing the brain mechanisms that control eating behavior become more sensitive to short -term hunger signs.

What for the behavior of eating? (Satiety signals)
The satiety signs are not necessarily the same ones that begin the behavior of eating. We do not stop eating so that we have recovered the different nutrients, but we do it long before this happens.
There are two main sources of satiety signals:
- Short -term signals: They are related to the immediate consequences of ingesting a specific meal and implying cephalic, gastric, intestinal and hepatic factors. They are signs that come from the immediate consequences of food. In these signals they can be involved receptors located on the face (which provide information on the flavor, smell, texture of food), in the stomach, the duodenum and the liver. These signals can constitute indicators that the food has been absorbed and is being digested.
- Long -term signals: They originate in the adipose tissue and allow to maintain body weight. These signals control calories by modulating the sensitivity of the brain mechanisms involved in the intake.
Ingest control implies an interaction between short -term signals and long term. If an individual has not been eating enough to maintain their weight, it will be less sensitive to satiety signals provided by food (short -term signals) and, therefore, will tend to eat more. If an individual has been eating in excess, and has gained weight, it will be more sensitive to short -term satiety signals provided by the same food.
 Central Nervous System (CNS): Structure, Functions and Diseases
Central Nervous System (CNS): Structure, Functions and Diseases Short -term satiety signals
Cephalic factors refer to Signs related to the taste, smell and texture of food. These anticipatory factors seem unimportant if we compare them with other signals from stomach, intestines or stages after food absorption.
For example, when a gastric fistula is implanted to a rat that prevents food from reaching the stomach, the animal eats continuously without showing signs of satiety. It seems that food must arrive at least in the stomach to trigger signs of satiety.
The fact that we stop eating long before digestion and food absorption take place in the intestine seems to indicate that signs originated in the stomach could be important in satiety mechanisms.
The distension of the stomach, as a result of the volume increase, constitutes a satiety signal.
In the stomach walls there are receptors that increase their activity proportionally to the stomach volume. These distension signals come through the vago nerve in the nucleus of the solitary tract (NTS) and in the dessert (AP) area of the brain trunk. This information reaches the hypothalamus and, lastly, in the cortex (where the perception of distension takes place).
When foods reach the stomach and intestines, these organs release different peptide hormones. These peptides could stimulate sensory fibers by providing the brain related to the amount of calories ingested.
The intestinal peptide cholecistocinin (CCK) is the best known example of these signals generated by the same eating and that control the amount of food we eat.
As digestion is being done, food is introduced into the duodenum where it is mixed with bile and pancreatic enzymes. The duodenum controls the stomach emptying rate through CCK secretion. When the receptors of the duodenum walls detect the presence of fats, CCK is segregated that provides an inhibitory signal of the stomach emptying to the duodenum.
But the CCK not only has a peripheral effect by controlling the emptying of the stomach, but acting on receptors located in the afferent fibers of the vago nerve carries information to the brain trunk. In the brain trunk, these Sinaptan sensory fibers with neurons that control reflections and digestive responses.
It is believed that the activity of the vago nerve produced by the CCK is synerly.
When the vagus nerve (vagotomy) is injured or the projection areas of the brain trunk are injured, the capacity of stomach distension decreases and of the CCK of inhibiting intake behavior.
The liver also provides satiety signals.
The administration of glucose in the abdominal cavity (which is captured by the liver and turned into glycogen) reduces the intake by sending signals to the brain through the vagus nerve.
Long -term satiety signals
It has been shown that the control of the intake is related to the maintenance of body weight. Most mammals tend to maintain their stable weight, Although there may be some variability in the amount of energy consumed and spent.
After a period of deprivation and, therefore, of weight loss, animals eat more until the levels of adiposity recover.
There are two compounds that could be defined as "adiposity signals" in blood, leptin (adipose tissue hormone) and insulin (pancreatic hormone).
Leptin function
Leptin is a hormone secreted by adipose cells in direct proportion to the amount of fat stored.
A slim individual secret less leptin and a thick secret more. Similarly, when an individual loses weight, plasma leptin levels decrease.
The importance of leptin as a sign of circulating adiposity in blood was revealed when Zhang et al leptin). The study of OB / OB / mice has also been important. OB / OB mice are not synthesized leptin due to a mutation in the OB gene. These animals are characterized by presenting extreme hyperfagia and obesity. When small amounts of leptin are administered to these animals, their body weight and food intake are normalized.
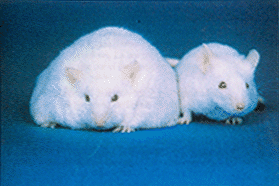
OB / OB / Ob and control mice. Source: John Pj Pinel (2001). Biopsychology. Madrid: Prentice Hall.
These results show that leptin constitutes a critical signal for the control of food and weight intake.
Under normal conditions, the amount of leptin released by adipose tissue in the blood correlates with the amount of body fat.
The relative stability of weight could be explained by the effects of leptin, Leptin already increases energy consumption, intake decreases.
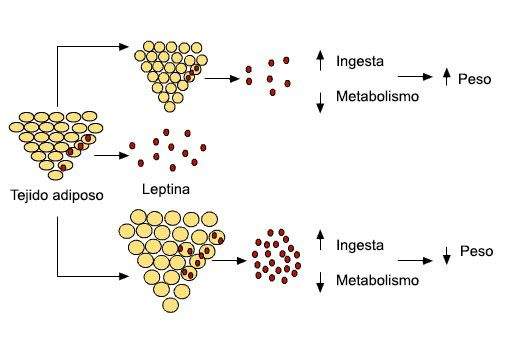
Relationship between the amount of fat stored in the adipose tissue, the release of leptin and body weight.
Leptin does not act only as an antiobesity hormone, but it seems to act as an indicator of the individual of the individual, informing whether energy reserves are sufficient or not or if there is an imbalance between the contribution and energy consumption. It could also be involved in behaviors such as the player, which represent great energy consumption and that, from an adaptive point of view, should only be implemented in those conditions in which the survival of the individual is guaranteed.
Leptin could be an important factor for the survival of the individual, acting as a generic informative sign to promote and conserve survival.
Where does the liberated leptin act?
We have receptors for leptin in the brain (especially in the hypothalamus), adenohypophysis, meninges, liver, lungs, thin intestine, gonads, adipose tissue, etc. Leptin acts both in peripheral tissues and in the central nervous system.
The effects of leptin on intake behavior and metabolism are related to its action in the hypothalamus.
 Frontotemporal dementia, what is it?
Frontotemporal dementia, what is it? Insulin function
Insulin is the hormone that makes it possible for tissues to use circulating glucose in blood.
Insulin secretion is directly related to adiposity levels.
Different data show the importance of insulin as a sign of adiposity in the brain:
- Animals with insulin deficit have hyperfagia. This hyperfagia disappears when insulin is managed directly to the brain.
- Insulin antibodies administration directly to the brain in normal animals increases intake.
In summary, the two hormones, insulin and leptin are signs related to adiposity that provide afferent information to the brain. Both hormones are released in blood, and by a transport system in the endothelial cells of the brain capillaries reach the CNS where they act on centers related to the control of energy homeostasis.
- Insulin and leptin are segregated in proportion to the stored fat content.
- These hormones act in the hypothalamus stimulating catabolic circuits (they promote energy spending and inhibit appetite) and inhibiting anabolic circuits (they stimulate intake and inhibit energy spending).
- Catabolic and anabolic circuits have opposite effects on the energy balance (difference between calories consumed and worn energy) that determines the amount of stored fats.
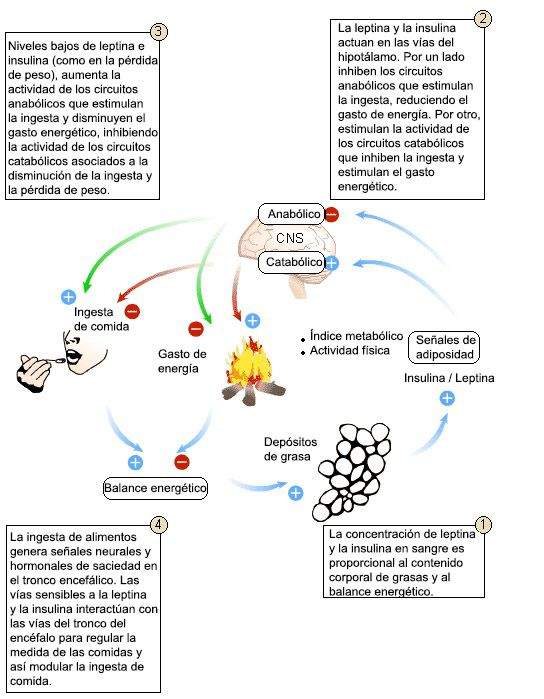
This model shows how changes in body adiposity are associated with compensatory changes in food intake. Leptin and insulin are signs of adiposity (secreted in proportion to the content of body fat) that act in the hypothalamus and stimulate the catabolic efferent pathways and inhibit anabolic. These routes have opposite effects on the energy balance (differences between calories consumed and spent energy) that determines the amount of energy stored in the form of fat.
- « Gestalt therapy and family constellations
- Trust in the Pareja Franqueza, Sincerity and Understanding »

